Imaging skeletal growth plates using in vivo multiphoton microscopy
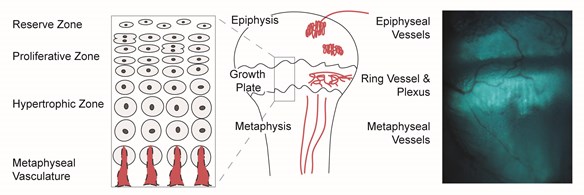
Multiphoton microscopy is an emerging technology for live animal imaging that offers exciting possibilities for the study of growth plate dynamics in vivo. In collaboration with colleagues at Cornell University, we developed a unique platform for imaging intact skeletal growth plates that we use to assess how systemic regulators arrive at and move within the cartilage matrix of the growth plate under various experimental conditions. This system provides a new mechanism for understanding the physiological regulation of bone growth through the ability to dynamically measure changes in molecular transport to the growth plate of a living animal (Fig. 3).
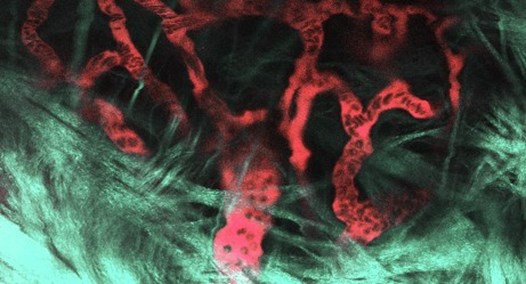
Figure 3. In vivo multiphoton image of blood vessels in the plexus surrounding the tibial growth plate of a live, anesthetized 5-week-old mouse. Vessels were visualized using a multiphoton microscope after an intravenous injection of fluorescein. Plasma is red and blood cells appear as dark shadows within the vessels. The collagen-rich perichondrium around the growth plate (green-gray pseudocolor) was visualized by second-harmonic generation (SHG), a robust signal from unstained collagen that is unique to multiphoton excitation. SHG allows collagenous structures to be identified without injecting stains or dyes. Imaging was done by Maria Serrat on a Leica TCS SP5 II Multiphoton Microscope housed in the Molecular and Biological Imaging Center at Marshall University (image modified from Serrat, 2014, Comprehensive Physiology, 4:621-55, Copyright 2014 American Physiological Society).
Using biologically-inert dextrans as size-proxies for systemic regulators, we have shown that heat can increase real-time entry of large molecules into growth plate cartilage in vivo (Serrat, Efaw, and Williams, 2014). We took this work a step further when we validated a model to measure uptake of fluorescently-labeled, biologically active IGF-I in growth plates of live, intact mice (Serrat and Ion, 2017) (Fig. 4). We are currently using this system to quantify IGF-I uptake in the growth plate under different environmental conditions (temperature and dietary influences).
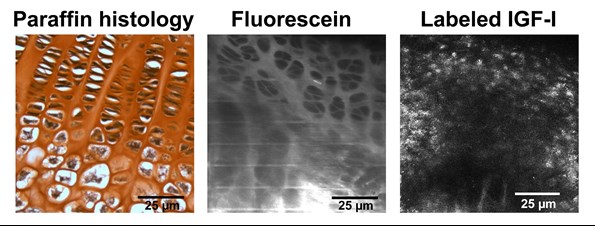
Figure 4. Comparison of growth plate visualization using standard postmortem histology (left) and in vivo multiphoton imaging of fluorescein dye (middle) and fluorescently-labeled, biologically active IGF-I (right). We developed techniques to quantify the transport of IGF-I into the growth plate of a living mouse. Chondrocytes (white with black nuclei in left panel) are shown as dark shadows when the growth plate is illuminated with fluorescein dye (middle). In contrast, fluorescence appears in a distinct pattern in the labeled IGF-I image (right), suggesting receptor-mediated localization to chondrocytes. Image on right from Serrat and Ion (2017).
Unilateral heating to lengthen extremities of growing mice
We recently developed and validated a novel unilateral heating model to increase extremity length on the heat-treated side of young growing animals. Our experimental system uses weanling mice (normal and dwarf models) exposed to a daily heating regimen to induce unilateral (one-sided) extremity growth. Mild 40C heat is applied to one side of the body for 40 minutes per day for two weeks as a unique, clinically applicable method to increase growth on the heat-treated side. This project complements our short-term in vivo imaging studies to determine the mechanisms underlying heat-enhanced bone elongation. Techniques employed in this project include microdissection; histology and immunostaining for bone morphology; fluorochrome bone labeling to measure elongation rate; protein assays to assess activation of growth stimulating pathways, and quantitative thermal imaging to measure heat gradients in the extremities (Fig. 5).
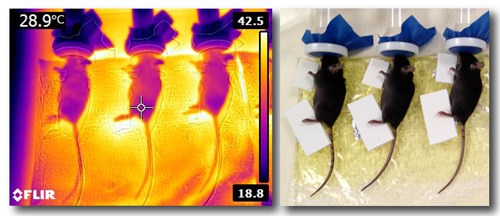
Figure 5. Limb heating experiments are being done in the Serrat Lab to study mechanisms underlying heat-enhanced bone elongation. Thermal image of juvenile mice on a heating pad (left) shows the temperature differential between heat-treated and non-treated sides. Limbs are equilibrated to 40C during the daily treatments. Digital image (right) shows foam separators that are used to keep the non-treated side at a cooler 30C. Images captured June 2014 by Holly Tamski and Maria Serrat.